Many women with ovarian cysts will have some concerns about the risk of ovarian cancer.
The risk of ovarian cancer is generally low. This low risk appears to be the same worldwide.
The quoted risk is 1.3% in a women's lifetime. It is generally lower (eg 0.2%) in young women and higher (eg 1.2%) in women above 60 years old.

CA125 or Cancer-Antigen-125 was identified in the 1980s as a test for ovarian cancer.
It is not the best test to diagnose ovarian cancer because many non-cancerous conditions can have a high CA125.
It is more effectively used to monitor treatment of certain cancers.
The normal CA125 is <35 U/ml. In the general population, there are healthy women with high CA125. The common benign, non-ovarian cancer conditions that increase CA125 are:-
CA125, by itself, is not accurate enough to screen for ovarian cancer. Hence none of the major health professional organisations recommend using CA125 as a screening test for ovarian cancer in the general population.
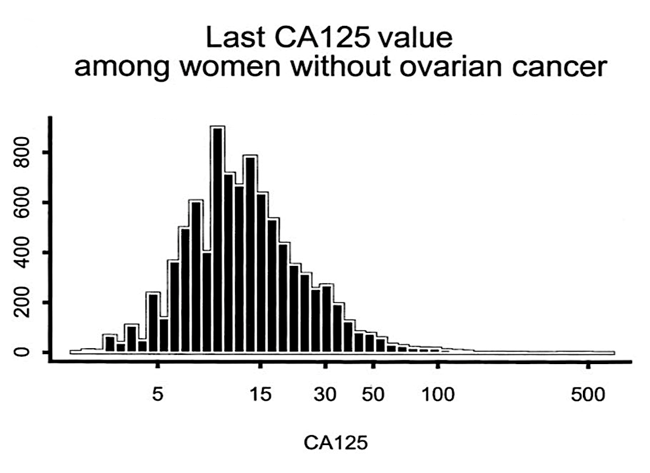
Even when ovarian cancer is present, the CA125 can be normal. In a study (Skates 2003) of 9233 women, it was found that:
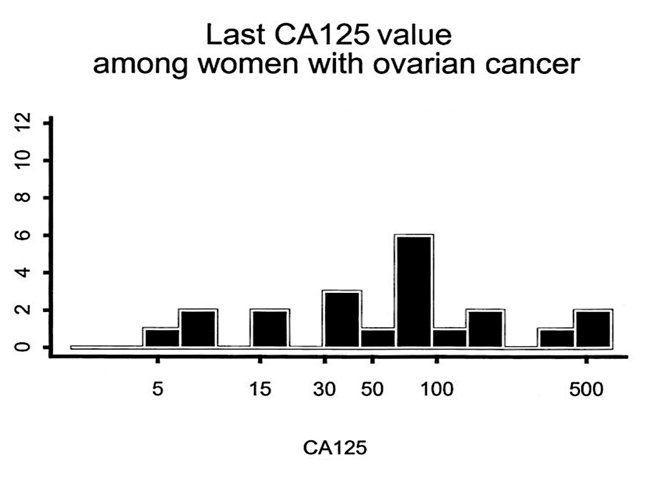
A recent study of CA125 and ovarian cancer (Funston 2019) found that CA125 may be detecting more ovarian cancers than thought.
Funston studied 50780 women and found 0.9% of them had ovarian cancer.
By age group, 0.4% of women <50 years and 1.2% of women >50 years had ovarian cancer.
In these 50780 women, CA125>76 in women <50 years and CA125>41 in women >50 years seem to predict the possibility of ovarian cancer.
Funston advised that these women should ideally have an urgent specialist referral for detailed assessment.

The International Ovarian Tumour Analysis (IOTA) Group has formulated ultrasound features suggestive of ovarian cancer. Based on these ultrasound features, the detection of ovarian cancer can be 95% sensitive and 91% specific. (Timmerman 2010)
Features of Benign Non-Cancerous Ovarian Cysts:
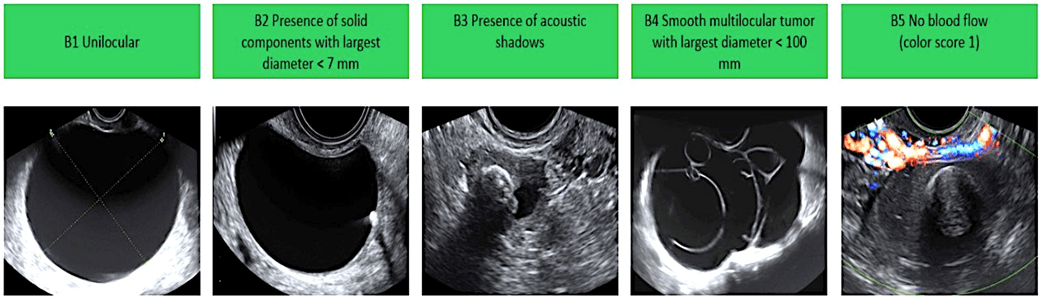
Features of Malignant Cancerous Ovarian Cysts:
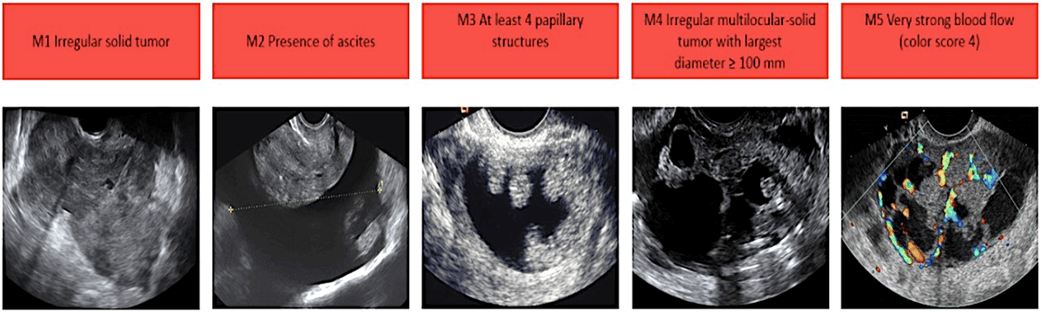
The Risk of Malignancy Index (RMI) uses CA125 and clinical features to assess the chance of ovarian cancer.
It cannot be used to diagnose ovarian cancer.
It is used to plan the detail treatment of ovarian cyst.
ROMA test is a computer calculation involving clinical factors (age >18yrs) and blood values of HE4 (Human Epididymis Protein 4) and CA125. ROMA test assesses the possibility of finding cancer in an existing ovarian cyst. It is not meant to be used as a screening test for ovarian cancer. ROMA test carries the following FDA Black Box Warning:-
Women with strong family history of cancer and women with BRCA-1 or BRCA-2 gene mutation are advised to have ovarian screening. Strong family history refers to the following relations:
Screening for ovarian cancer involves CA125 and pelvic ultrasound every 4-6 months. Although screening cannot prevent ovarian cancer, they can possibly diagnose ovarian cancer earlier, hopefully at Stage 1. You can read more about ovarian cancer screening by downloading this article "Cancer and the Ovaries. Understanding ovarian cancer and screening methods"
 Top
Top